Hydrogen production by electrolysis. / 电解制氢。
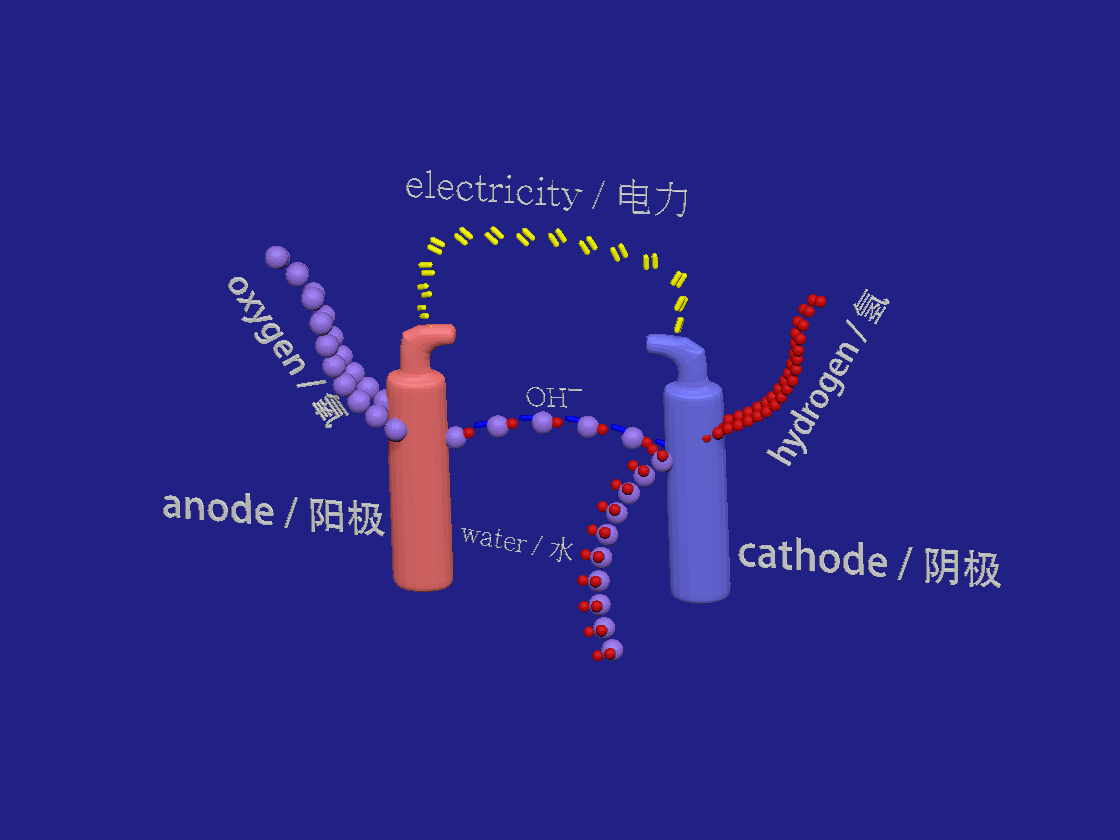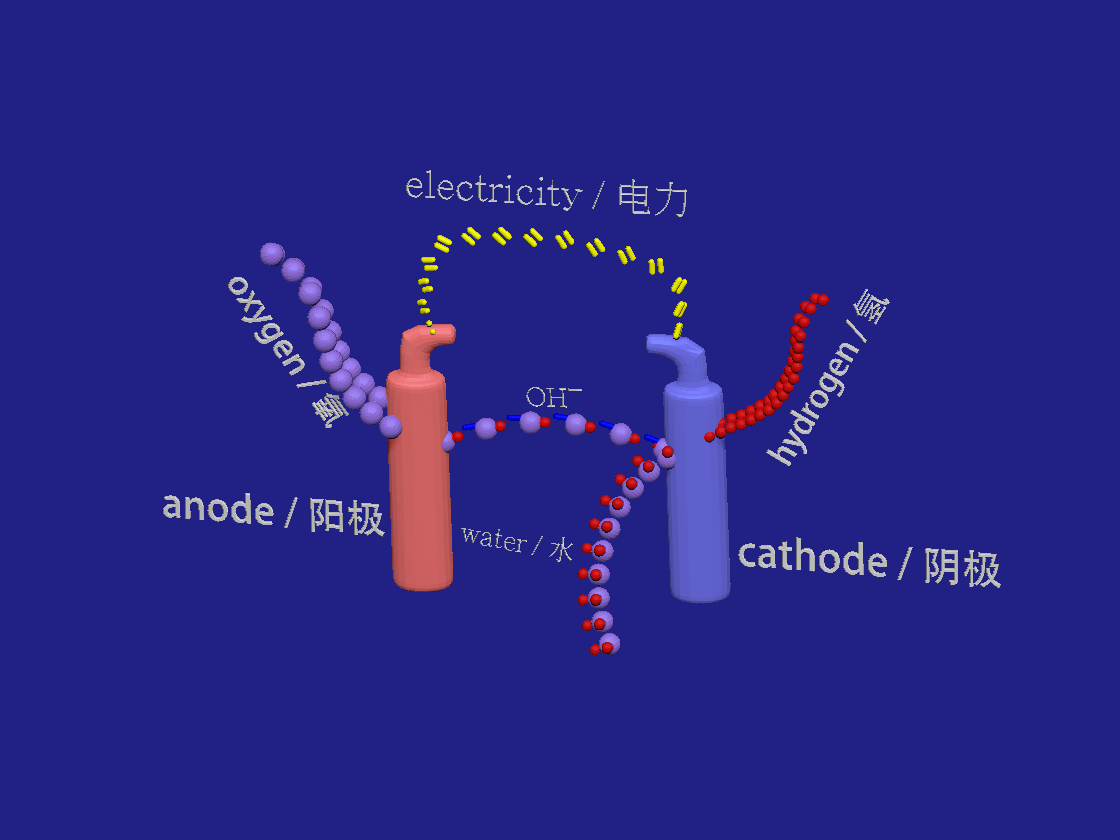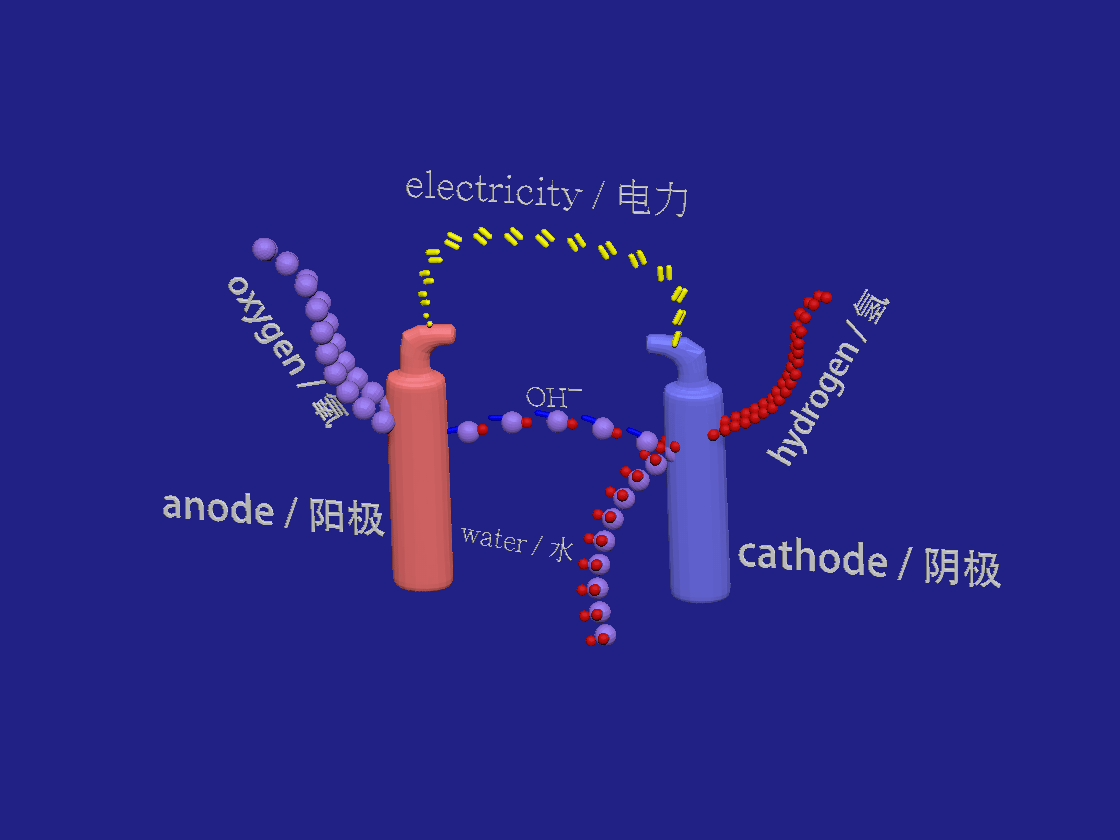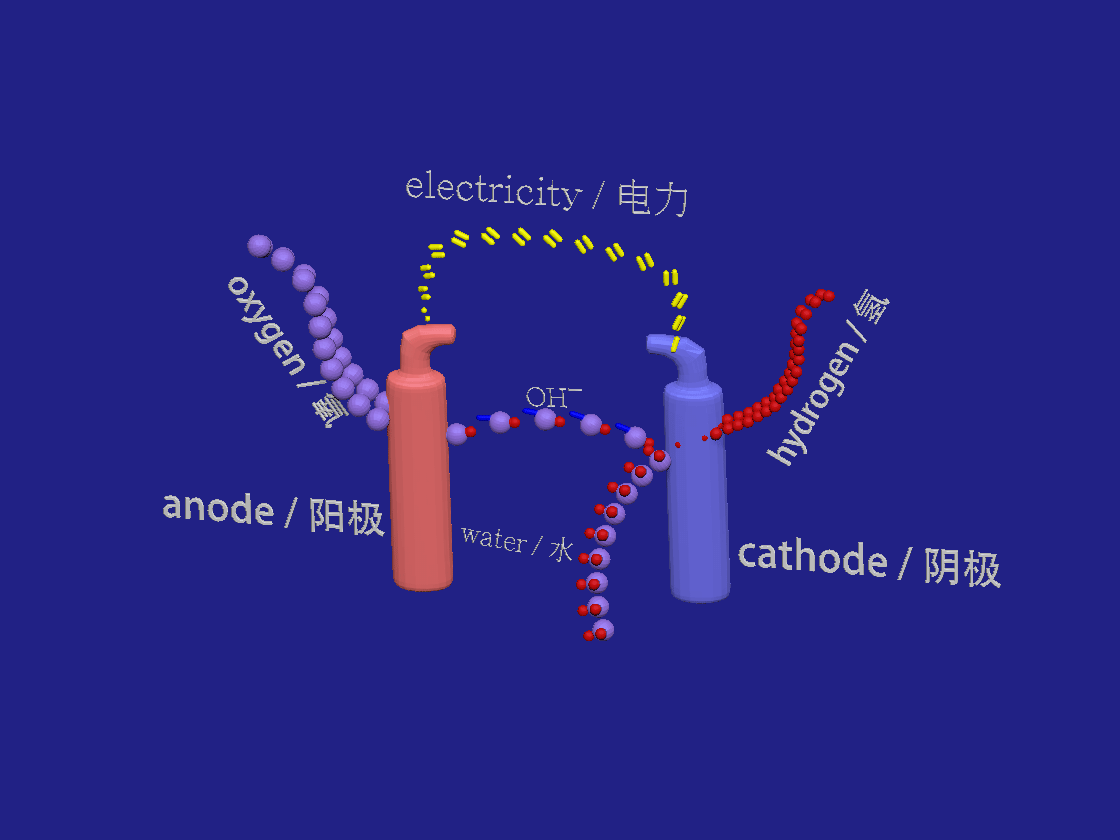
2H2O + 2e- > H2 + 2OH-
2OH- > H2O + 1/2 O2 +2e-
In the akalyne electrolyser the negative cathode looses electrons to the aqueous solution. Water breaks down into hydrogen and hydroxide ions. At the positive anode the electrons are absorbed by the negative hydroxide ions which are so oxidised to form water and oxygen.
在芳烃电解槽中,负极将电子释放到水溶液中。水分解成氢和氢氧根离子。在正阳极,电子被氢氧根负离子吸收,氢氧根离子被氧化形成水和氧。
Hydrogen production - steam methane reforming method / 制氢-甲烷蒸汽重整方法
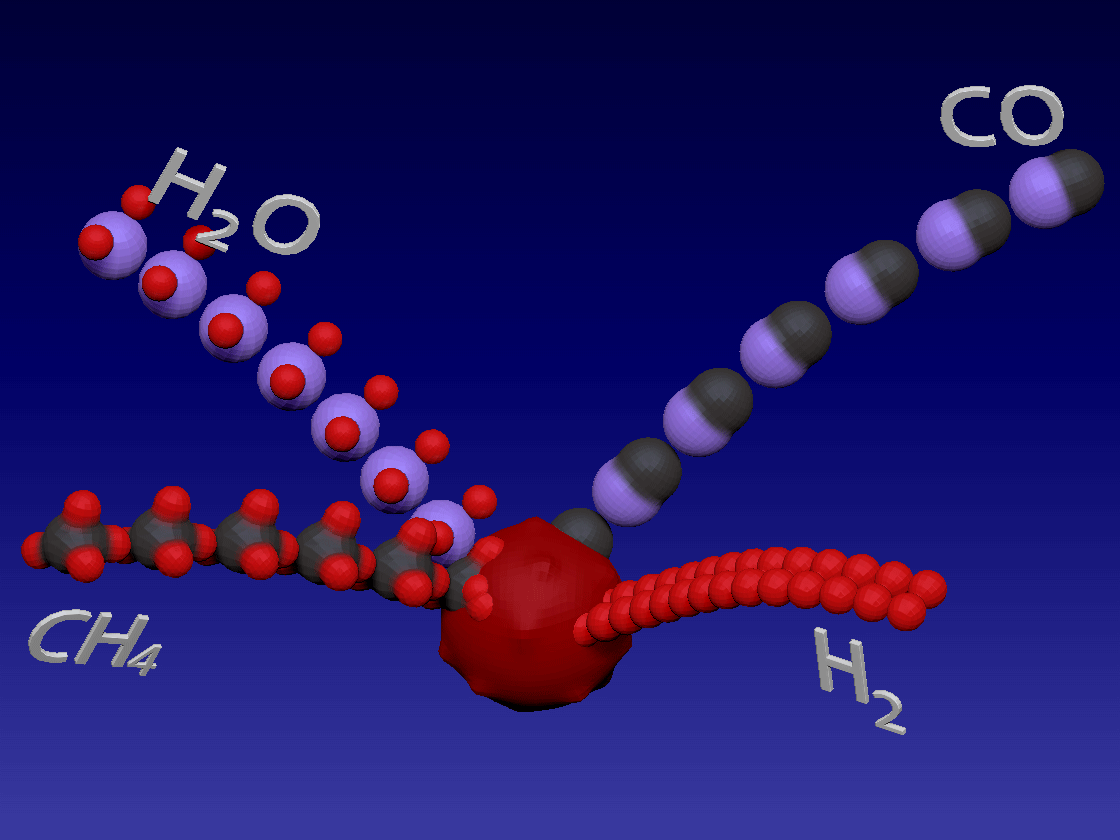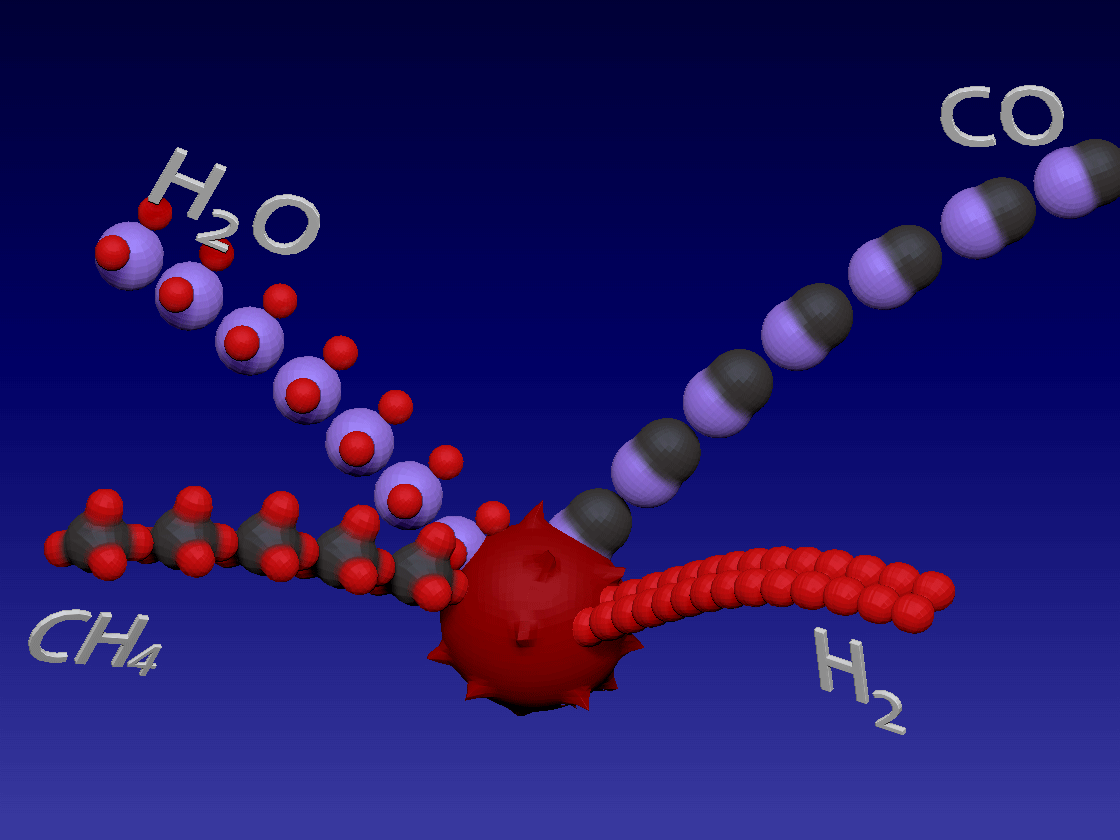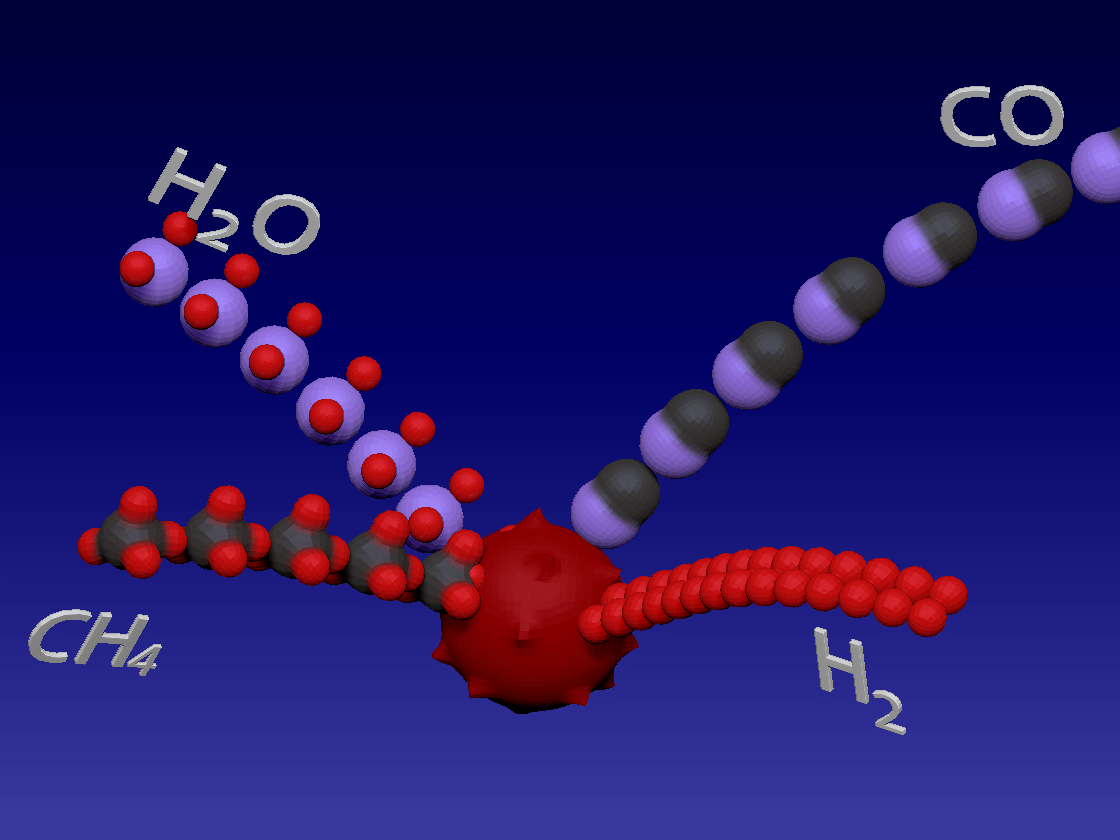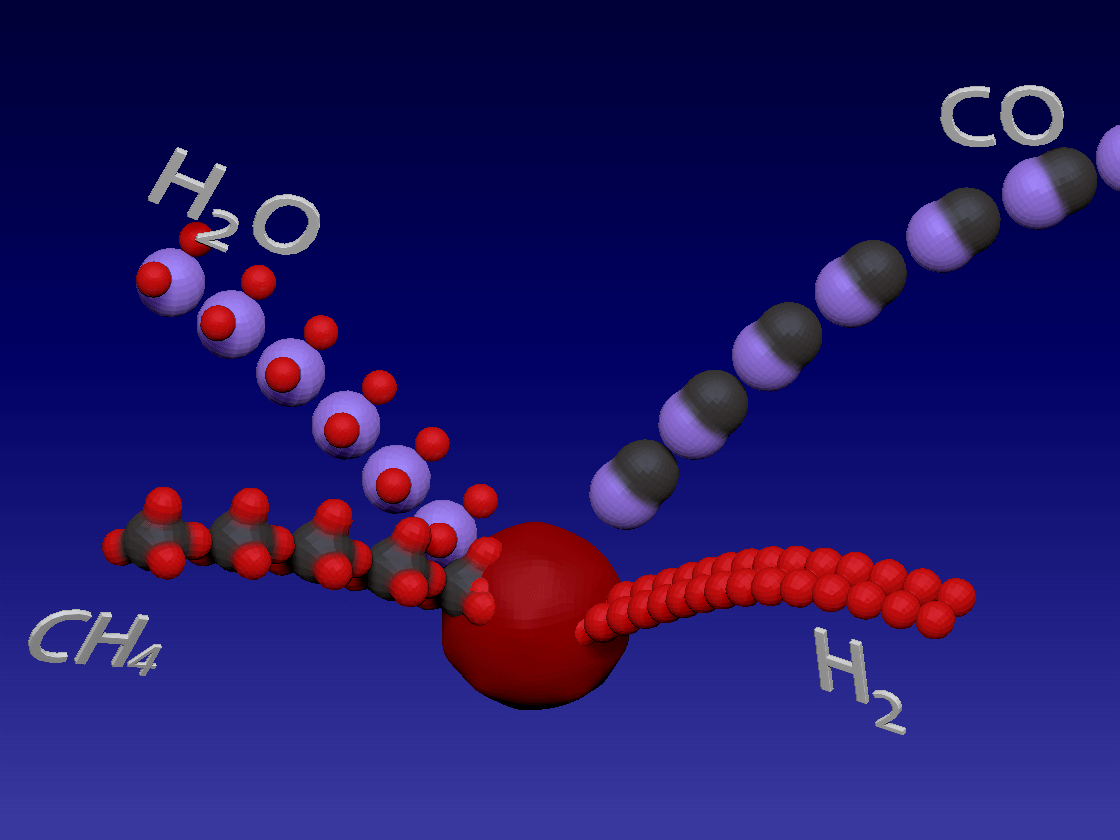
CH4 + H2O > CO + 3H2
Methane and water steam react in high temperature and in the presence of a catalyst to produce hydrogen and carbon monoxide. Heat needs to be added during this reaction (endothermic reaction)
甲烷和水蒸气在高温下和催化剂的存在下反应生成氢和一氧化碳。该反应(吸热反应)期间需要添加热量
Hydrogen production - Gasification / 氢气生产-气化
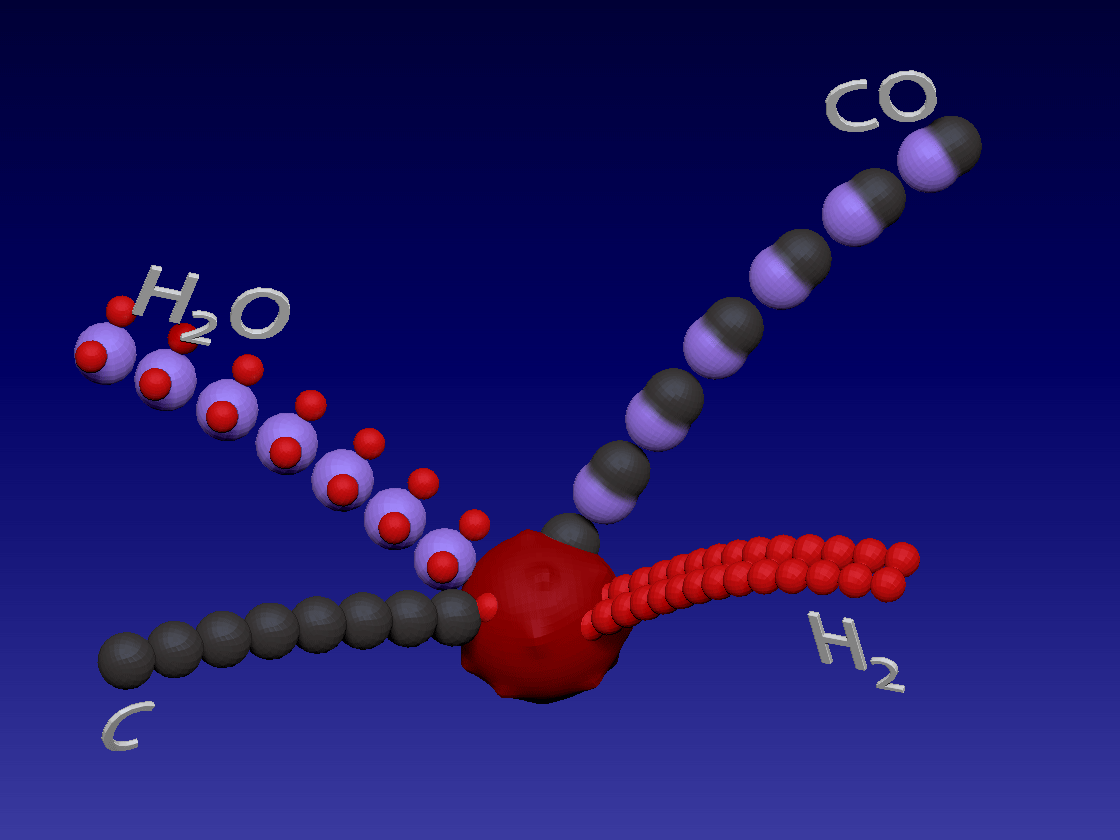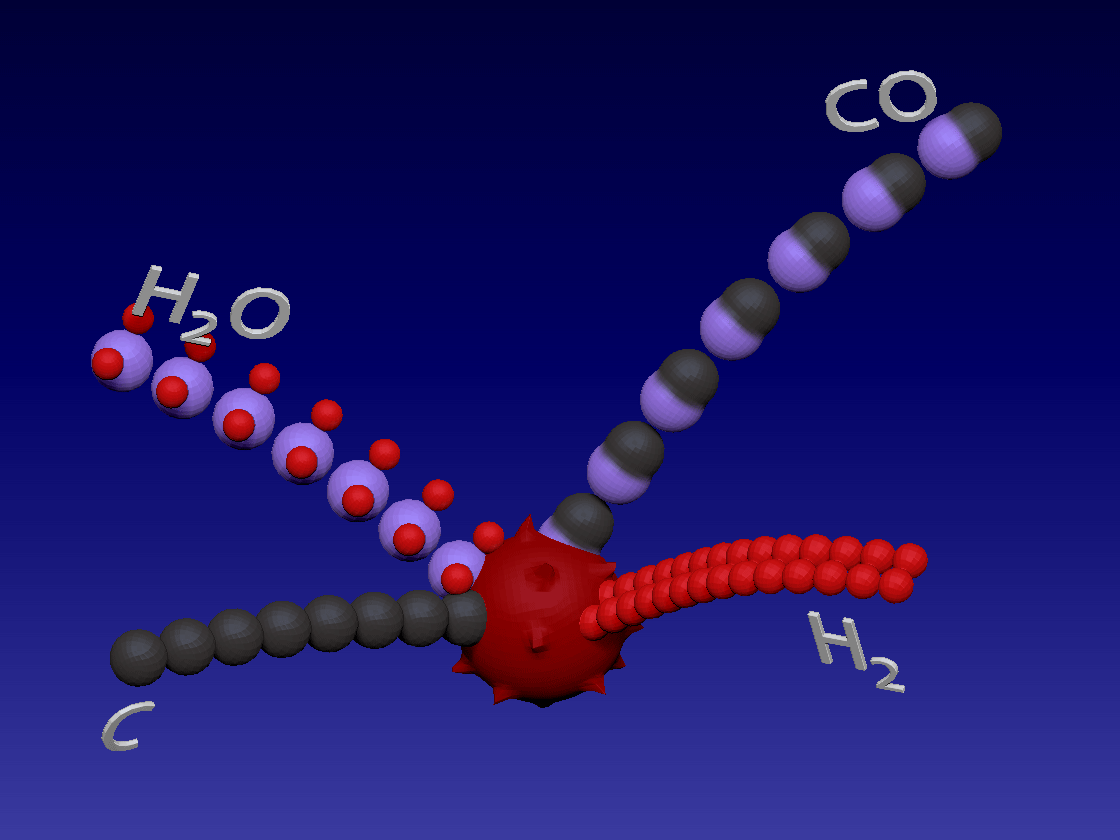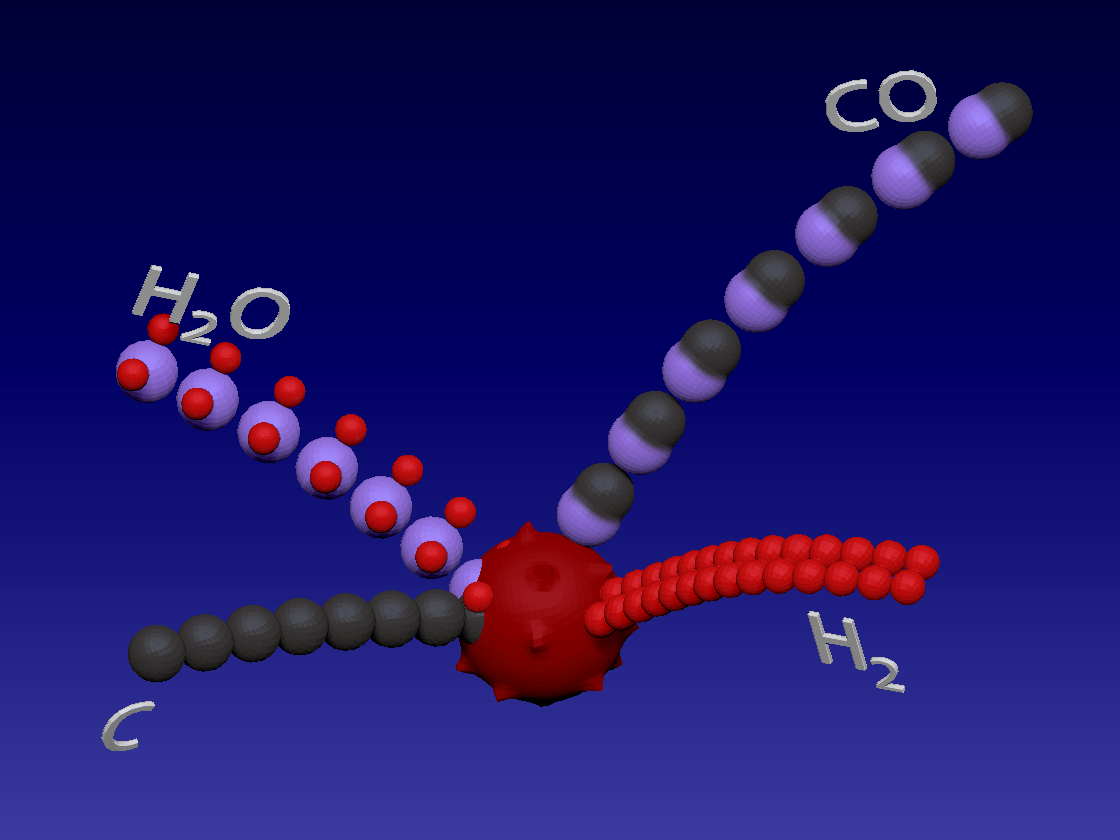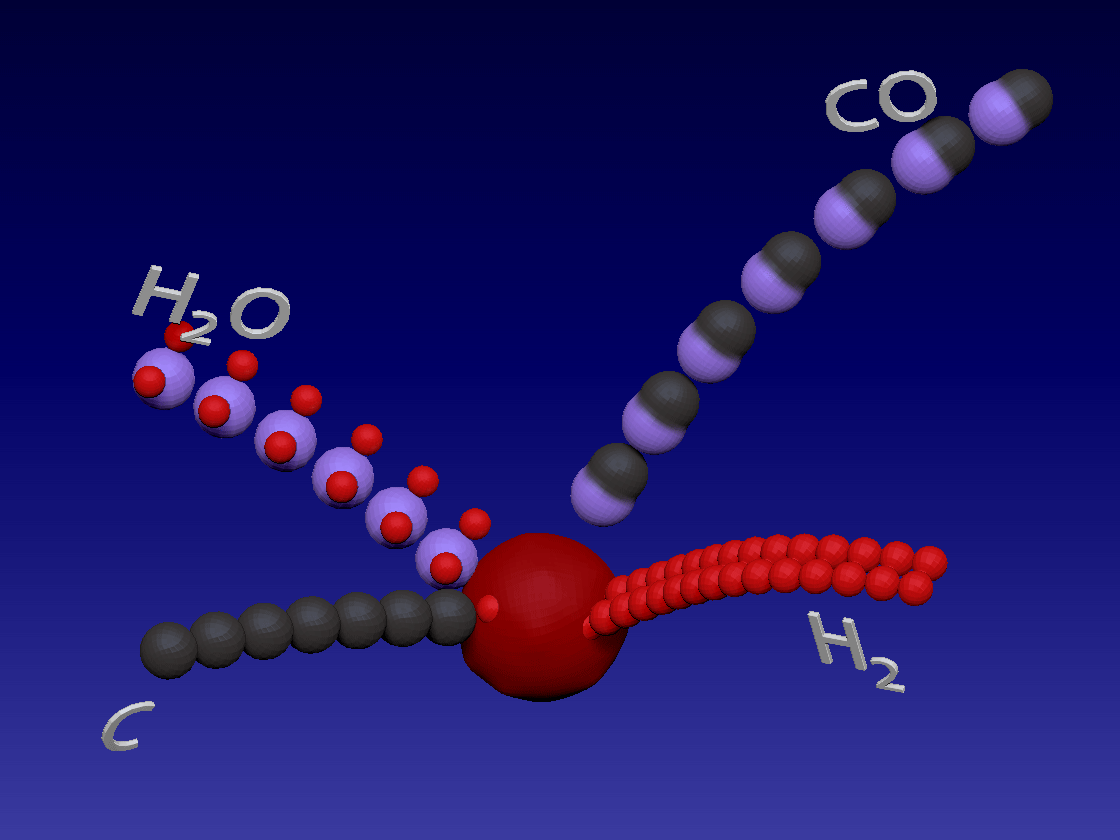
C + H2O > CO + H2
Carbon (coal) reacts with water in high temperature to produce carbon monoxide and hydrogen. This is also an endothermic reaction.
碳(煤)在高温下与水反应生成一氧化碳和氢气。这也是吸热反应。
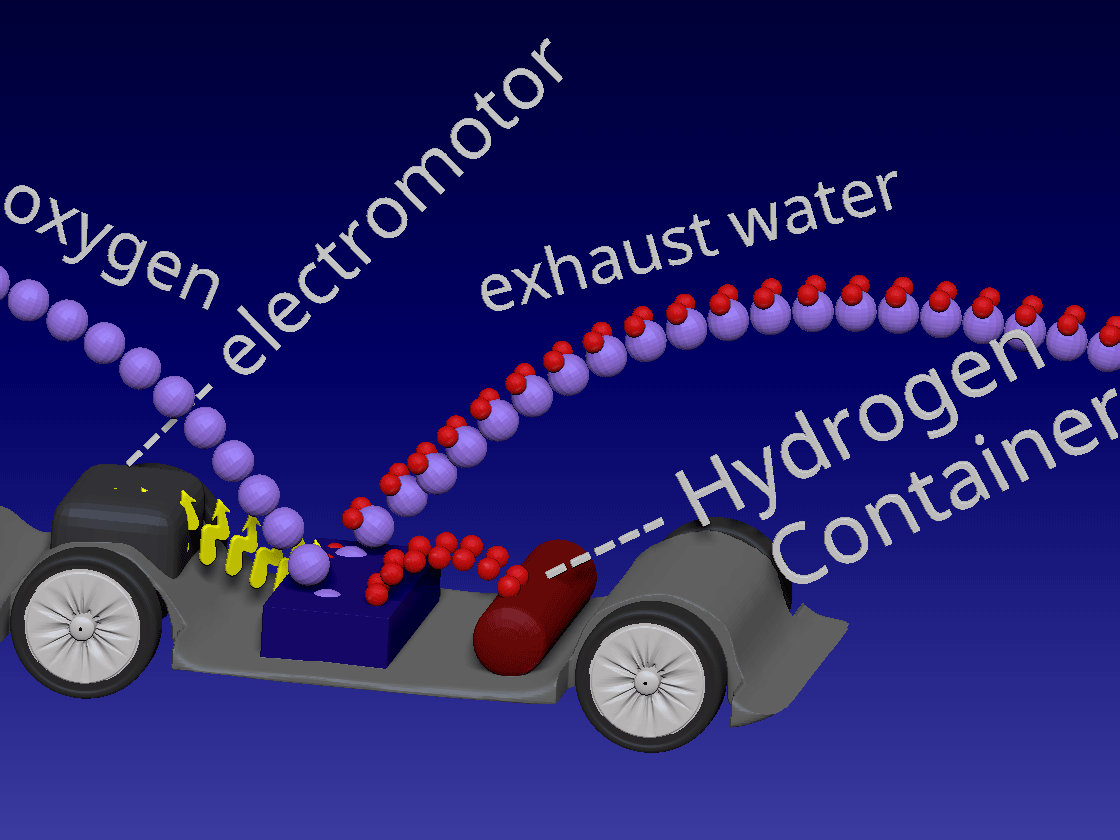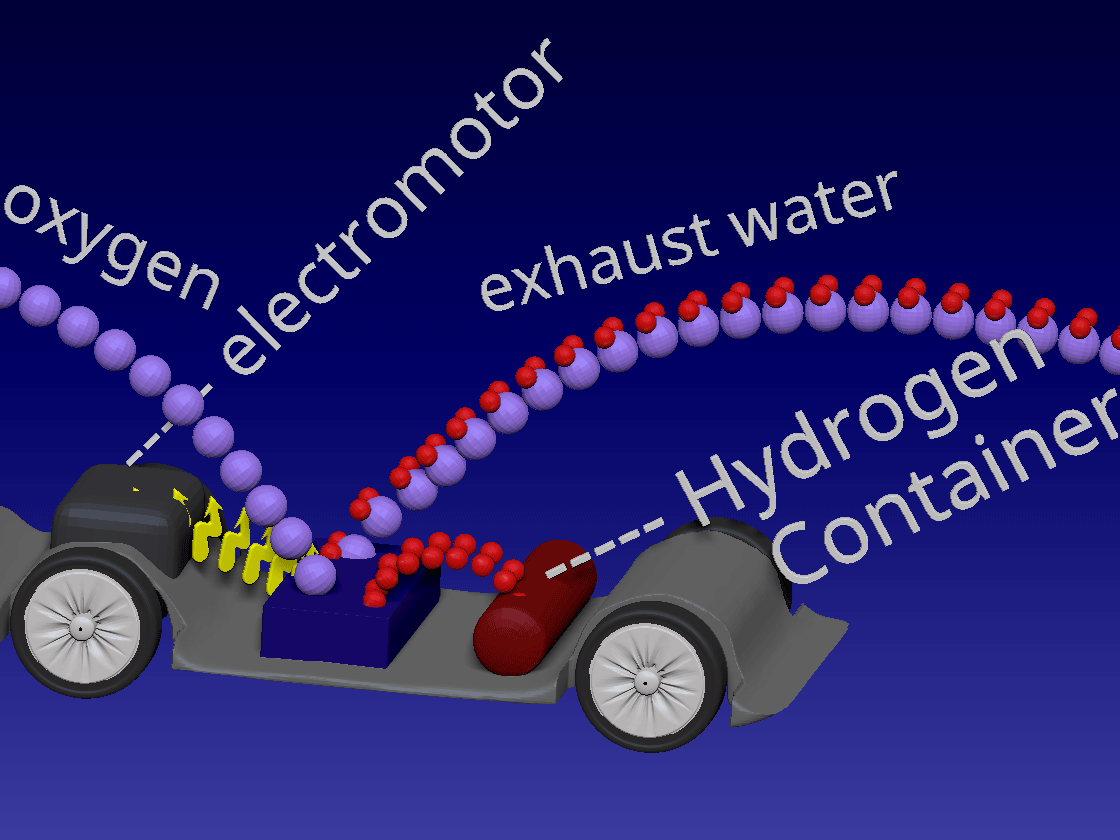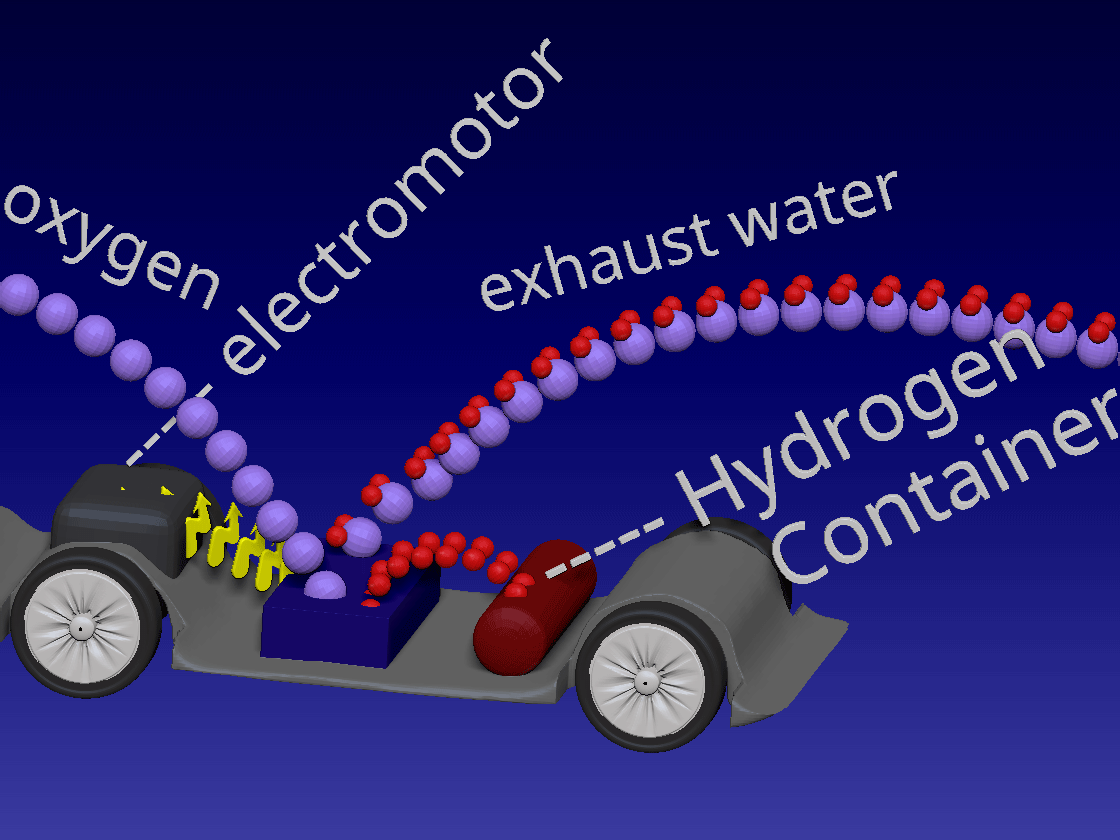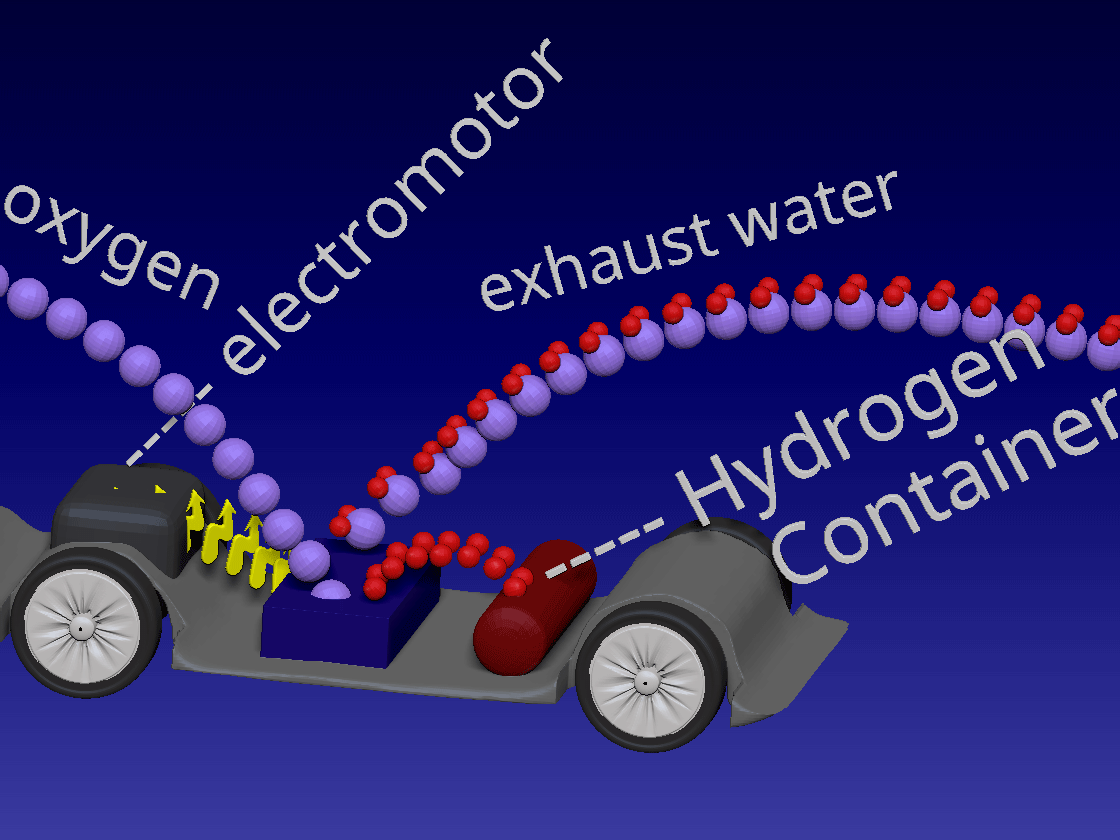
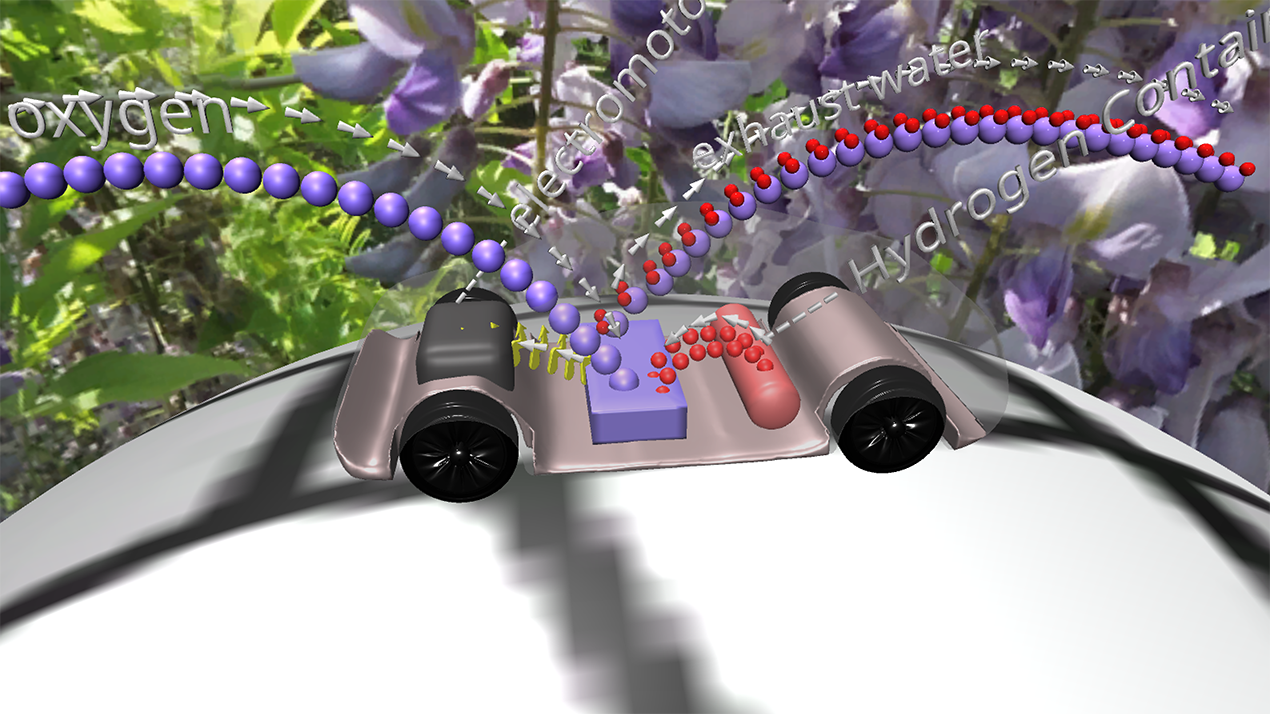
Fuel Cell / 燃料电池
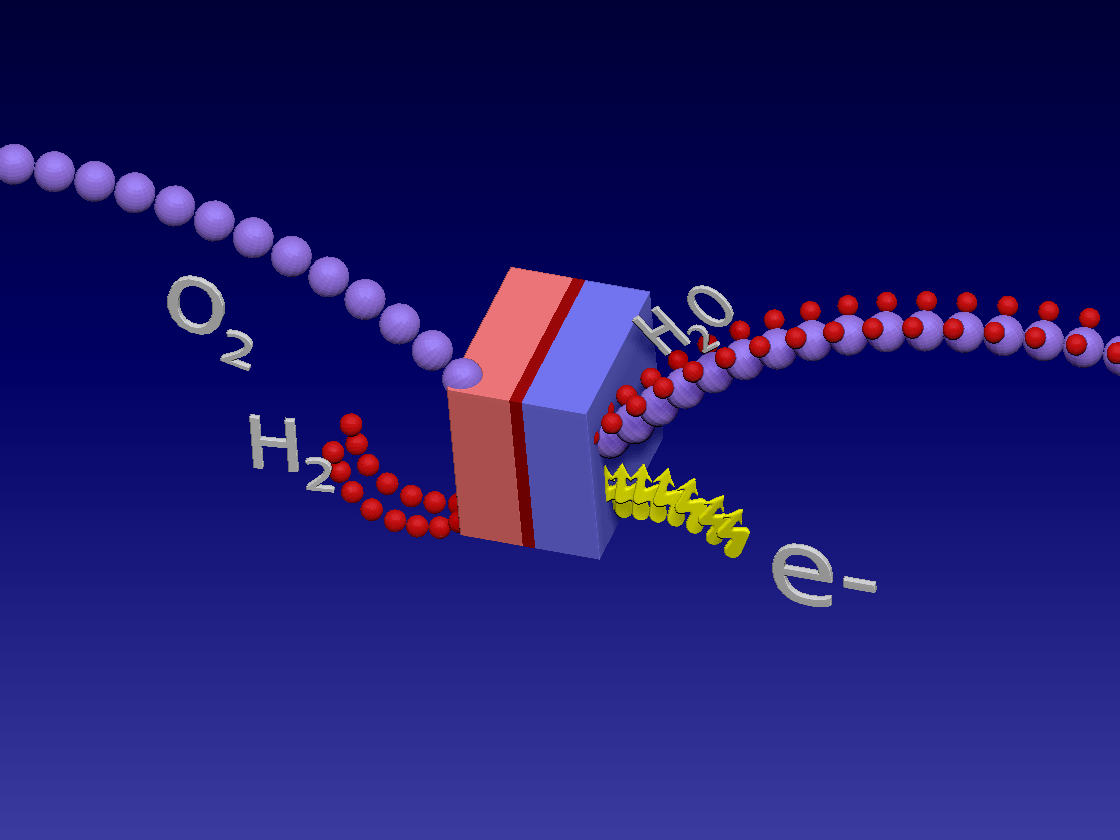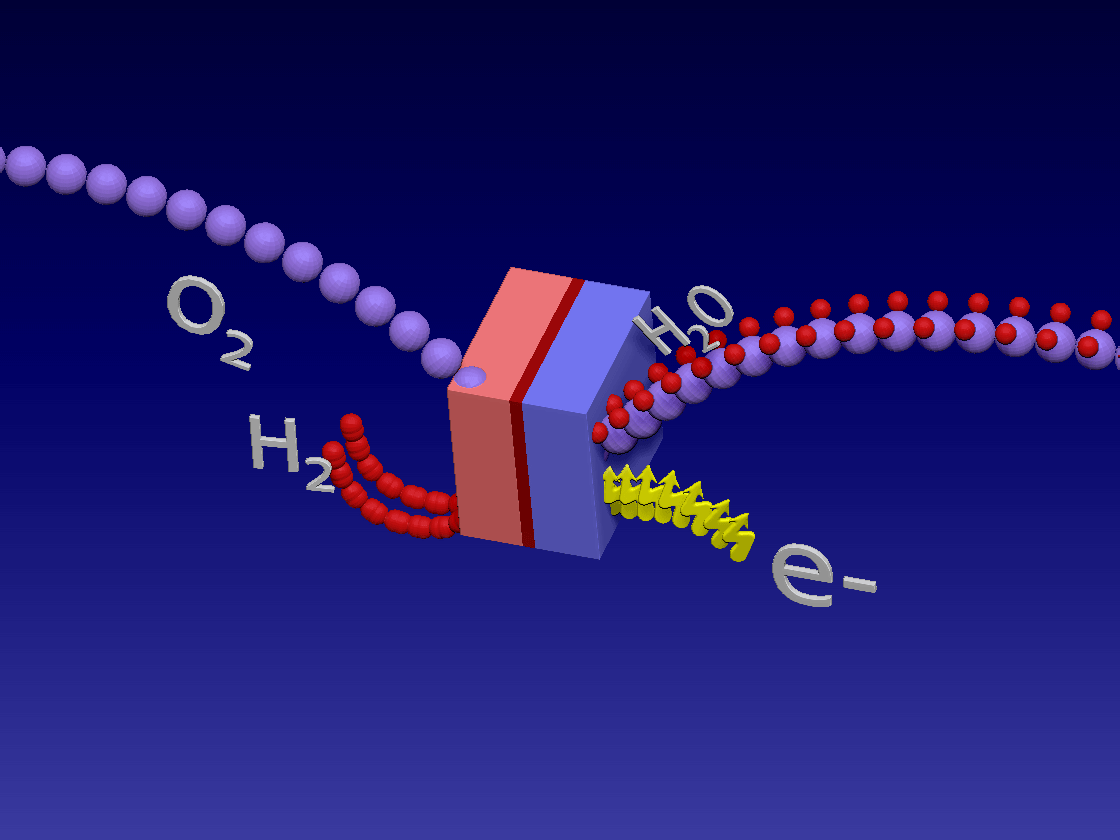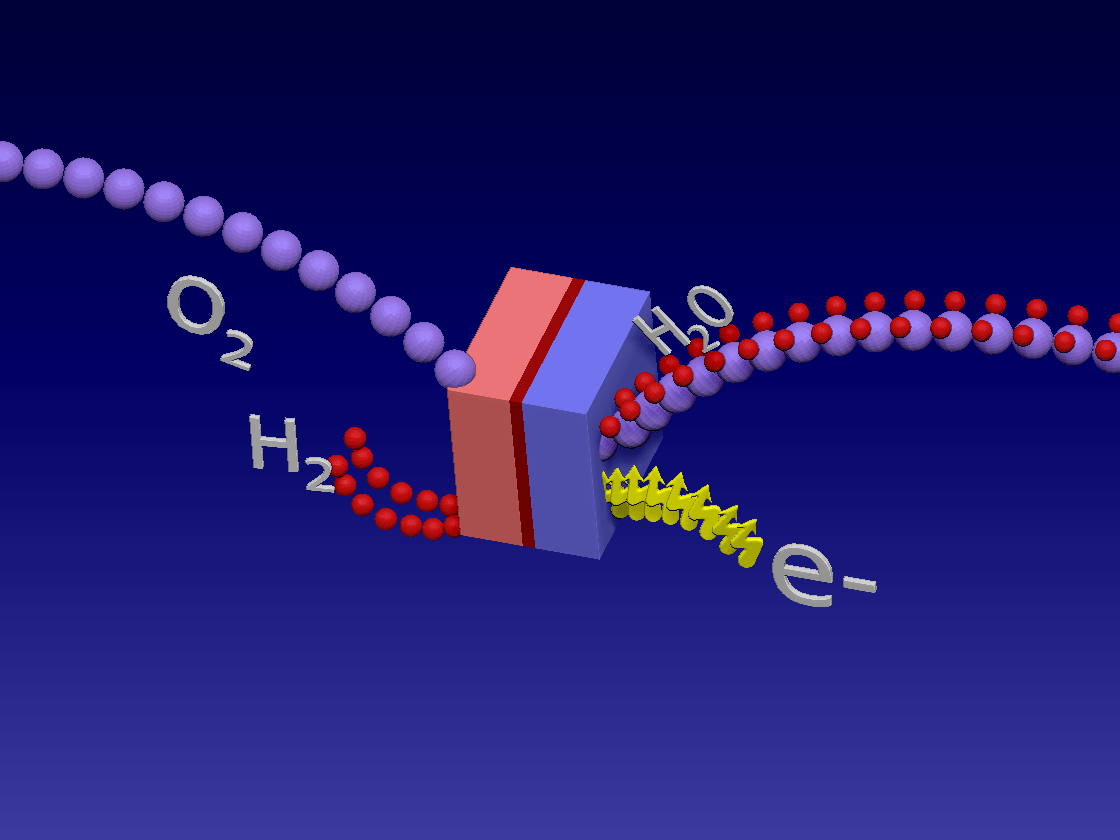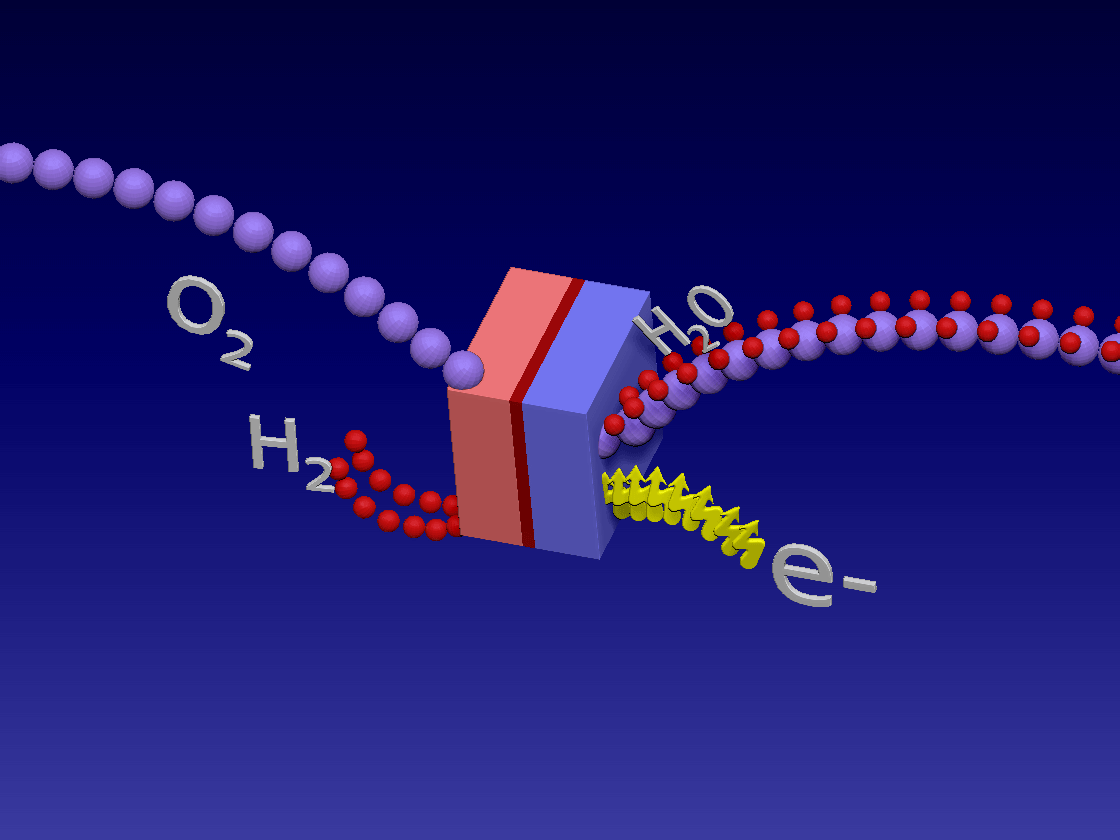
来自储罐的氢气进入正极。空气中的氧气向下流到负极端子。
正极(红色)由铂制成,铂是一种贵金属催化剂,旨在加速燃料电池中发生的化学反应。当氢气原子到达催化剂时,它们分裂成氢离子(质子)和电子。带正电的质子被吸引到负极(蓝色)并通过电解质流向负极。电解质是由特殊的聚合物(塑料)薄膜制成的中间薄膜,只有质子才能通过。电子为驱动汽车的电动机供电。